A chemical reaction involves either electron removal, electron addition, or electron sharing. The path that a specific element will take in a reaction depends on where the electrons are in the atom and how many there are.
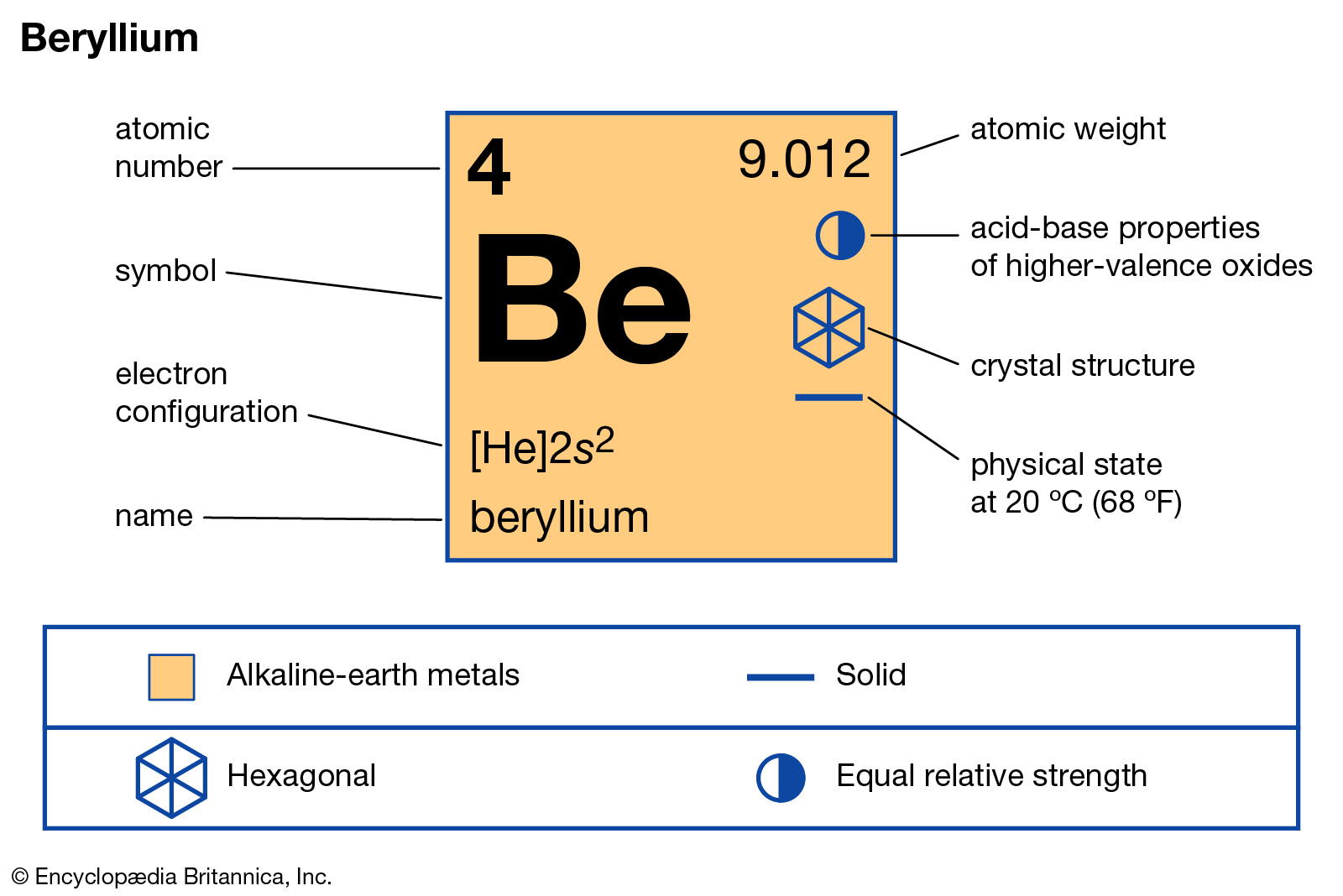
The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z. Electron configuration of Beryllium is He 2s2. Possible oxidation states are +1; +2. Beryllium's most common isotope is 9 Be, which contains 4 protons and 5 neutrons. It makes up almost 100% of all naturally occurring beryllium and is its only stable isotope; however other isotopes have been synthesised. In ionic compounds, beryllium loses its two valence electrons to form the cation, Be 2+. A beryllium atom with two valence electrons would have the electron dot diagram below. Its valence electron shell is 2s 2 2p 1 so it has three valence electrons. Berylliums electrons fills up the first orbital with 2 electrons in the second orbital. Since 1s can only hold two electrons the remaining 2 electrons for be go in the 2s orbital.

Valence Electrons
In the study of chemical reactivity, electrons in the outermost principal energy level are very important and so are given a special name. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the (1s) sublevel are called inner-shell electrons and are not involved directly in the element's reactivity, or in the formation of compounds. Lithium has a single electron in the second principal energy level, and so we say that lithium has one valence electron. Beryllium has two valence electrons. How many valence electrons does boron have? Recognize that the second principal energy level consists of both the (2s) and the (2p) sublevels, and so the answer is three. In fact, the number of valence electrons goes up by one for each step across a period, until the last element is reached. Neon, with its configuration ending in (2s^2 2p^6), has eight valence electrons.
Summary
- Valence electrons are the outer-shell electrons of an atom.
- Valence electrons determine the reactivity of an atom.
Contributors and Attributions
Beryllium Fluoride Valence Electrons
Name: Beryllium: Symbol: Be: Atomic Number: 4: Atomic Mass: 9.012 atomic mass units: Number of Protons: 4: Number of Neutrons: 5: Number of Electrons: 4: Melting. Beryllium has two valence electrons. List the number of valence electrons in each element. Beryllium has two valence electrons in its 2s shell, so its electron dot diagram is like that of. How many valence electrons does a sodium, silicon, beryllium, and oxygen atom have?
CK-12 Foundation by Sharon Bewick, Richard Parsons, Therese Forsythe, Shonna Robinson, and Jean Dupon.
Learning Objectives
- Define valence electron.
- Be able to indicate valence electrons when given the electron configuration for an atom.
What makes a particular element very reactive and another element non-reactive?
A chemical reaction involves either electron removal, electron addition, or electron sharing. The path a specific element will take depends on where the electrons are in the atom and how many there are.
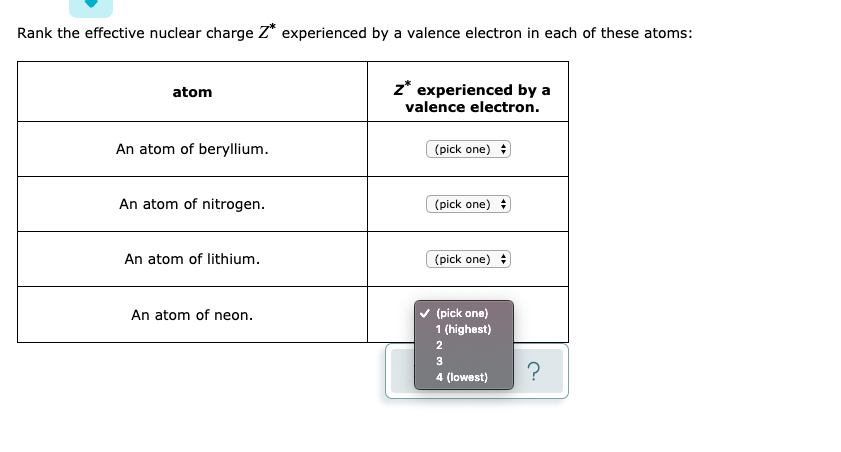
| Element Name | Symbol | Atomic Number | Electron Configuration |
| Lithium | Li | 3 | 1s22s1 |
| Beryllium | Be | 4 | 1s22s2 |
| Boron | B | 5 | 1s22s22p1 |
| Carbon | C | 6 | 1s22s22p2 |
| Nitrogen | N | 7 | 1s22s22p3 |
| Oxygen | O | 8 | 1s22s22p4 |
| Fluorine | F | 9 | 1s22s22p5 |
| Neon | Ne | 10 | 1s22s22p6 |
In the study of chemical reactivity, we will find that the electrons in the outermost principal energy level are very important and so they are given a special name. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements listed above, the two electrons in the 1 s sublevel are called inner-shell electrons and are not involved directly in the element’s reactivity or in the formation of compounds. Lithium has a single electron in the second principal energy level and so we say that lithium has one valence electron. Beryllium has two valence electrons. How many valence electrons does boron have? You must recognize that the second principal energy level consists of both the 2 s and the 2 p sublevels and so the answer is three. In fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. Neon, with its configuration ending in s2p6, has eight valence electrons.
Summary
- Valence electrons are the outer-shell electrons of an atom.
- Valence electrons determine the reactivity of an atom.

Practice
Use the link below to answer questions about valence electrons:
Review
- Define valence electron.
- Define inner shell electron.
- How many valence electrons are there in fluorine?
- What are the 2s electrons in nitrogen?
- How many inner shell electrons are there in beryllium?
Glossary
- inner-shell electrons: Those electrons that are not in the outer shell and are not involved in the reactivity of the element.
- valence electrons: The electrons in the highest occupied principal energy level of an atom.
Beryllium Valence Electrons
Show References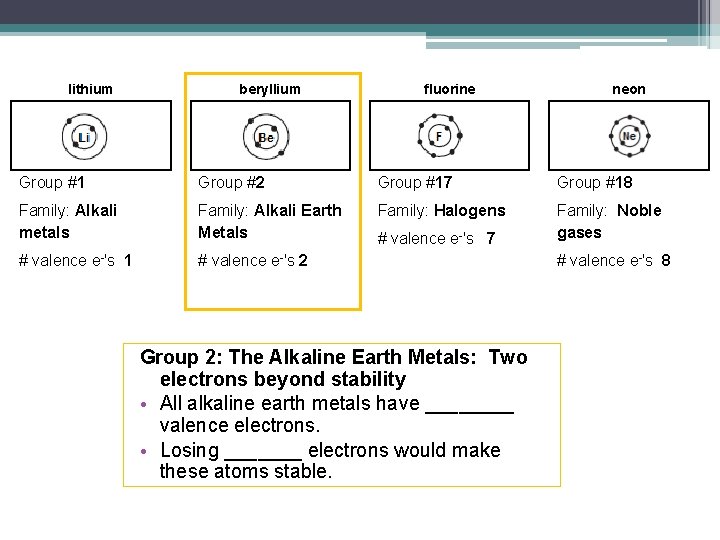
References
Beryllium Ion Valence Electrons
- User:Chemicalinterest/Wikipedia. http://commons.wikimedia.org/wiki/File:Cobalt_carbonate.JPG.

Comments are closed.